The lawyers at Saiontz Kirk PA. The impurity detected in the finished drug product is N.
 Blood Pressure Medication Valsartan Manufactured By Aurobindo Recalled Over Cancer Concerns Fda
Blood Pressure Medication Valsartan Manufactured By Aurobindo Recalled Over Cancer Concerns Fda
If youve recently filled Valsartan or Valsartan HCTZ with Humana Pharmacy we wanted to let you know that certain manufacturers have issued a recall of select lots of the medicine.

Valsartan hctz recall. The voluntary valsartan recall specifically applies to certain valsartan and valsartan hydrochlorothiazide valsartan HCTZ produced or distributed by Major Pharmaceuticals Solco Healthcare and Teva Pharmaceuticals Industries. Taking valsartanhydrochlorothiazide with this drug can cause low salt levels. Valsartan is used to improve your survival chances after having a heart attack.
Solco and Teva valsartanhydrochlorothiazide tablets were affected by the recall. Aurobindo refused to provide updated. It is also used to treat heart failure and reduce the risk of heart failure.
Valsartan is used for the treatment of high blood pressure for the treatment of heart failure and to reduce cardiovascular mortality following. Are reviewing potential Valsartan and Valsartan HCTZ recall lawsuits for individuals who used potentially contaminated pills and developed. Get an alert when a recall is issued.
Details about the products affected by the valsartan recall products are available through the FDA. The affected lots are being recalled due to an unexpected impurity in the manufacturers active pharmaceutical ingredient API. Aurobindo Pharma USA Inc.
Product Description NDC Lot. However many blood pressure medications containing Valsartan have been recalled. The recalled amlodipinevalsartanHCTZ tablets were distributed nationwide.
Side Effects Adverse Reactions. Several common drugs that contain valsartan used to treat high blood pressure and heart failure have been recalled in the United States due to an impurity in the drug that poses a potential. 627 rows In July 2018 the US.
Food and Drug Administration FDA. Valsartan is used to lower high blood pressure. PRESS RELEASE - FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity FDA-published testing methods to provide options for.
Is conducting a voluntary recall of 80 lots of Amlodipine Valsartan Tablets USP Valsartan HCTZ Tablets USP and Valsartan Tablets USP to the consumer level due to the detection of trace amounts of an unexpected impurity found in the finished drug product. Taking valsartanhydrochlorothiazide with this drug can increase your risk of gout. The affected lots are being recalled due to an unexpected impurity in the manufacturers active pharmaceutical ingredient API.
On August 17 2018 the FDA announced a voluntary consumer-level recall of several lots of Torrents amlodipinevalsartanhydrochlorothiazide HCTZ tablets due to the presence of an impurity N-nitrosodimethylamine NDMA. We wanted to let you know that certain manufacturers have issued a recall of select lots of Valsartan ValsartanHCTZ combination product and ValsartanAmlodipine combination product. In July 2018 the FDA announced a voluntary recall of several drugs containing valsartan used to treat high blood pressure and heart failure because of contamination with an impurity the potentially cancer-causing chemical N-nitrosodimethylamine or NDMA.
Detailed information on the products is available at httpswwwfdagovdownloadsDrugsDrugSafetyUCM615703pdf. The expanded recalled batches are as follows. We understand that this medicine is.
The recalled valartan and valsartan HCTZ pills all contained ingredients from a supplier in China which has been contaminated with N-nitrosodimethylamine NDMA for years. Solco and Teva discontinued valsartanhydrochlorothiazide tablets in July 2018. Other drugs affected by the valsartan recall include losartan and irbesartan.
 Las Vegas Area Doctor Explains Recall Of Common Blood Pressure Medication
Las Vegas Area Doctor Explains Recall Of Common Blood Pressure Medication
 Fda Recalls All Amlodipine Valsartan Medications Due To Cancer Risk
Fda Recalls All Amlodipine Valsartan Medications Due To Cancer Risk
 Solco Healthcare Recalls Valsartan And Valsartan Hctz Tablets
Solco Healthcare Recalls Valsartan And Valsartan Hctz Tablets
 2 High Blood Pressure Medications Added To Recall Of Heart Drug Abc News
2 High Blood Pressure Medications Added To Recall Of Heart Drug Abc News
 Nationwide Recall Of Valsartan Blood Pressure Medication Expanded Daic
Nationwide Recall Of Valsartan Blood Pressure Medication Expanded Daic
Valsartan Canada Recall 1 3 Per Tablet
 Torrent Pharmaceuticals Recalls Valsartan Amlodipine Hctz Tablets
Torrent Pharmaceuticals Recalls Valsartan Amlodipine Hctz Tablets
Valsartan Hctz Used For Valsartan With Diuretic
 Valsartan Recall 4 Things Patients Should Know Cnn
Valsartan Recall 4 Things Patients Should Know Cnn
Has There Been A Recall On Valsartan 39 Usd
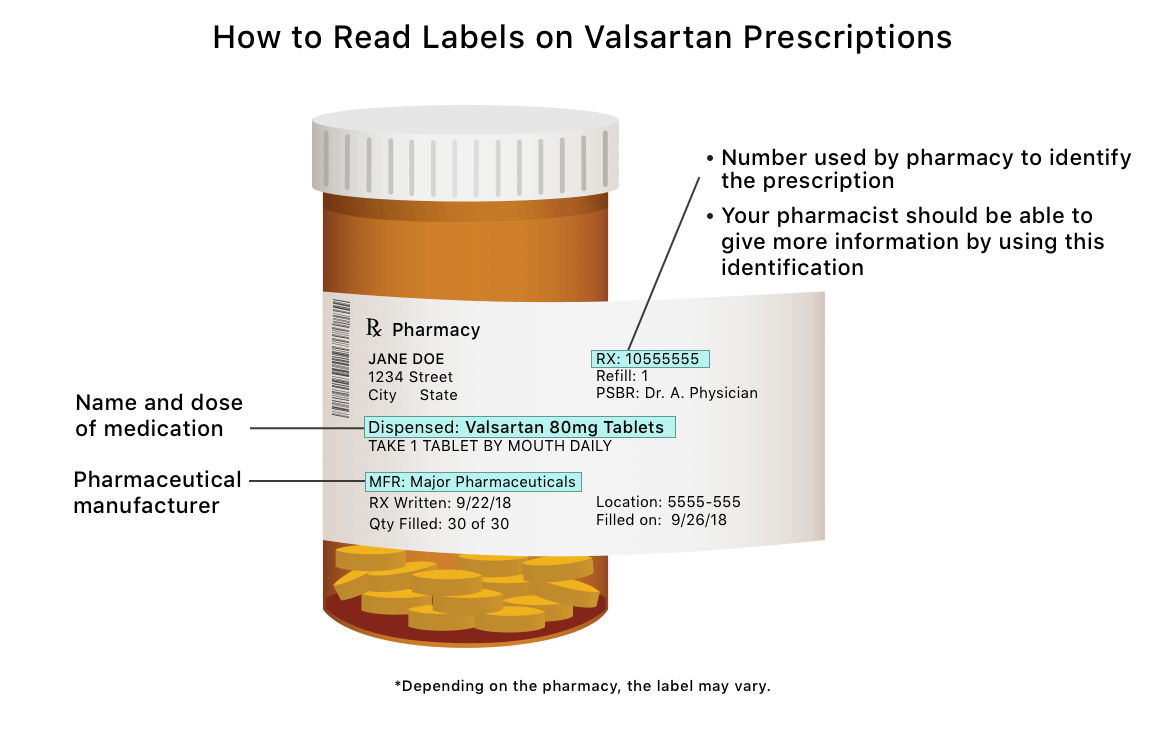 Valsartan Recall Why Is The Fda Recalling Valsartan
Valsartan Recall Why Is The Fda Recalling Valsartan
 Blood Pressure Meds Recalled Over Contamination Concern Wfmj Com
Blood Pressure Meds Recalled Over Contamination Concern Wfmj Com
 Check Your Medicine Blood Pressure Rx Recalled Rxwiki
Check Your Medicine Blood Pressure Rx Recalled Rxwiki


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.