The 340B CONFIDENT service determines 340B eligibility after a claim adjudicates. This list is in addition to the quarterly MEF posted on the 340B Office of Pharmacy Affairs Information System.
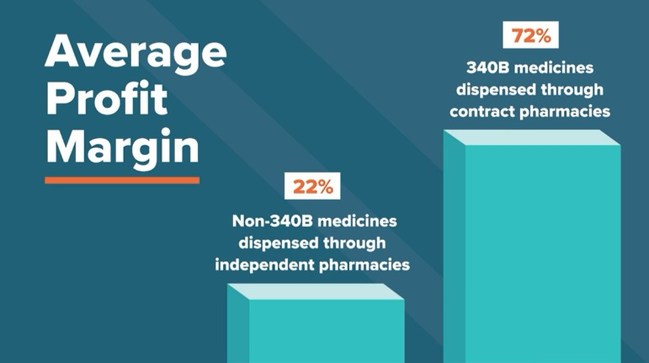 New Analysis Shows Contract Pharmacies Financially Gain From 340b Program With No Clear Benefit To Patients
New Analysis Shows Contract Pharmacies Financially Gain From 340b Program With No Clear Benefit To Patients
Controversy around the 340B Program picked up in 2020 as several major drug manufacturers including AstraZeneca and Eli Lilly began refusing discounts for 340B drugs if they were to be dispensed through contract pharmacies.

List of 340b pharmacies. As a reminder 340B contract pharmacies earn per-prescription fees paid by the 340B entity instead of typical dispensing spreads and fees. List of 340b hospitals by state. Contract Pharmacies The 340B program currently represents 11 percent or 674B of total US drug sales and has grown five times faster than the growth rate of the overall drug market.
Contract pharmacies must register for the 340B Program and be listed on the 340B OPAIS prior to dispensing 340B drugs on a covered entitys behalf. Over the past 10 years the number of covered entities ie hospitals clinics eligible for 340B discounts increased 34 to 13000. We recognize that circumstances surrounding disaster relief efforts warrant flexibility for entities eligible for participation in the 340B Program.
Perhaps the most misreported number in the 340B debate is the number of pharmacies that have contracts with 340B healthcare providers. Please use these handouts to highlight the importance of 340B to your region. This expansion of contract pharmacies in the 340B program is the primary concern for manufacturers as contract pharmacies.
Takeda Pharmacy Partner Forcing 340B Pricing Opt-inOpt-out Choice on Entities In Pharma Industry May 11 2021 458 Views Tom Mirga Takeda business partner Foundation Care is making 340B covered entities declare on a form whether they will opt in or opt out of 340B pricing on eligible product purchases as a condition for obtaining a Takeda leukemia drug. Public Health Emergency Declaration by the Secretary. That number currently stands at 13833.
There are no additional steps required. Appendix A lists the fee arrangements in agreements that the GAO reviewed. If you do not find the exact resolution you are looking for then go for a native or higher resolution.
During that same time period the number of contract pharmacies on the 340B eligibility list has skyrocketed 1700 to 23000 3. The Contract Pharmacy Search Criteria page displays when you select Search Contract Pharmacies on the 340B home page. Covered entity search filters on are the left side of the page CP filters are.
Contract pharmacies must carve-out Medicaid ie not use 340B drugs for. 340B contract pharmacies Disclaimer Please be advised that the following information is provided for reference purposes only. Download this image for free in High-Definition resolution the choice download button below.
One component driving the tremendous growth of the 340B program is contract pharmacies. The confusion comes from the way the Office of Pharmacy Affairs tracks contract pharmacies in its database. This program was established by the government to ensure hospitals provide access and services to the most vulnerable patients.
Therefore eligible entities in Texas may immediately enroll for the 340B. Prescriptions written or drugs dispensed in clinical sites listed in OPAIS or in conjunction with other services listed as in scope on form 5C of the health centers Scope of Project are eligible for 340B pricing As discussed in Section 5B8 a clinical site must be approved. The deadline to comply is Oct.
Hospitals across the country depend on the 340B program to increase access to care for low-income andor rural communities. Covered entities are responsible for ensuring compliance of their contract pharmacy arrangements with all 340B Program requirements. According to some reports as much as 25 of 340B and Medicaid discounts overlap 2.
Drug manufacturers are obligated to deliver its covered outpatient drugs to contract. List of 340b contract pharmacies is important information accompanied by photo and HD pictures sourced from all websites in the world. Search criteria can be selected individually or in combination to allow you to narrow your search results to a manageable number of records.
The 340B Program Enables UHS to Provide Care and Services All UHS Retail Pharmacies participate in a Federal Program known as 340B. In addition other large drug manufacturers began requesting that covered entities provide more detailed reports on their 340B. On July 27 2020 drug manufacturer Sanofi sent a letter to all 340B providers requesting that they submit the same data as Merck except for Sanofi to 340B ESP and stating explicitly that if a 340B provider did not comply they would refuse to permit any Sanofi drugs to be shipped to its contract pharmacies.
For background see Section 105 of our 2018 Economic Report on US. Dont forget to bookmark list of 340b contract pharmacies. Your contracted pharmacies serve patients just as they normally do and you can be confident that there will be no disruption in serving your customers.
January 04 2021 - New guidance from HHS has clarified that hospitals and other covered entities in the 340B Drug Pricing Program are entitled to discounts for covered outpatient drugs even if they use contract pharmacies to distribute the drugs to patients. Pharmacies and Pharmacy Benefit Managers. Searching for Contract Pharmacies.
HRSA has the authority to audit covered entities for compliance with 340B Drug Pricing Program 340B. We have created handouts that list each 340B hospital by state and Congressional district. 3 The size and scope of 340B today resembles nothing like the program created by Congress in 1992.
The content provided has neither been endorsed nor approved by the Office of Pharmacy Affairs and is not dispositive in determining participatory status in or. Instead of listing these brick-and-mortar stores only once the agency counts the number of contracts between a 340B provider and a pharmacy. This information does not constitute legal advice and should not be construed as such.
Here is how I characterize. Contact Elise Cranston with any questions at. The program is fully funded by the pharmaceutical companies not by the government.
To carve in drugs dispensed or administered at a hospital location or an entity-owned pharmacy an entity must inform OPA of its decision to carve in and ensure that all numbers it uses to bill 340B drugs to FFS Medicaid ie national provider identifier NPI andor state-specific billing numbers are listed in OPAs Medicaid Exclusion File.