CBS 2s Jim Williams reports on trials in Chicago that involve not one but two doses of the Johnson Johnson COVID vaccine. The research firm also announced they are recruiting people to take part in another two-shot COVID-19 vaccine trial for Johnson and Johnson.
 Canada Signs 2 More Deals For Coronavirus Vaccines Voice Of America English
Canada Signs 2 More Deals For Coronavirus Vaccines Voice Of America English
In a deal with the US government Johnson Johnson priced its vaccine at about 10 R150 per dose much lower than the Pfizer 19 per dose and Moderna 25 to 37 per dose vaccines Forbes reported.

Johnson and johnson vaccine trial sign up. The Johnson Johnson vaccine trial started September 23 with a plan to recruit 60000 patients in eight countries including the United States. Heres what you need to know. The clinical trial is being conducted to evaluate the efficiency and safety of the Johnson and Johnson COVID-19 vaccine.
Johnson Johnson is expanding a clinical trial for its COVID-19 vaccine to include minors. For more information about trial click here. Johnson Johnsons single-shot coronavirus vaccine protected against symptomatic and asymptomatic infection and prevented hospitalization and death in all participants 28 days after vaccination.
However women younger than 50 years old especially should be aware of the rare risk of blood clots with low platelets after vaccination and that other COVID-19 vaccines are available where this risk has not been seen. Any vaccine that you are offered you should strongly consider taking it Schroeder said. Eligible participants will receive two doses of either the investigational vaccine or a placebo with the doses given 57 days apart.
If you received a JJJanssen vaccine. The vaccine is currently being administered to adults in America under an emergency use authorization granted in December. CNNJohnson Johnsons Covid-19 vaccine candidate begins Phase 3 trials in the United States today.
The approval process is likely to take months. The Johnson Johnson vaccine candidate known as JNJ-78436735 is delivered by a harmless virus called Adenovirus 26 a human virus uncommon. The study participants are 18 years old and older including significant representation from those that are over age 60 said Johnson Johnson.
Trials for the single-dose vaccine will include up to 60000 adult participants at nearly 215. Trial participants must be between the ages of 60 and older. We asked two experts whose job it is to test vaccines to help demystify the clinical trial processstep by step.
Anyone interested in volunteering may sign up at this link on the Henry Ford website. While it appears to be not as effective at preventing COVID-19 as Pfizer or Moderna those who took it and still caught the virus. November 05 2020.
But what does this step in the process really mean. Johnson Johnson just announced the launch of a Phase 3 clinical trial for its COVID-19 vaccine candidate. Unlike the Moderna and Pfizer vaccines Johnson Johnsons inoculation is a single-dose vaccine which means its distribution could enable more people to be fully vaccinated sooner.
The next phase of the trials will include minors aged 12 years old to 17 years old. Administration of the Johnson Johnson Covid-19 vaccine can be resumed after a pause due to reports of a very rare type of blood clot according to health officials. However the results of the clinical trial show that the vaccine is significantly less effective than the two vaccines currently being administered in the US.
The Johnson and Johnson vaccine is the latest to get FDA approval. If you are interested you can sign up here. To volunteer you just need to be 18 or older and I good or stable health.
To find out what this. In its statement Friday Johnson Johnson said. DETROIT Henry Ford Health System has been selected as a site for Janssen Pharmaceutical Companies of Johnson Johnsons Phase 3 clinical research study ENSEMBLE trial to evaluate the safety and efficacy of the Janssens investigational COVID-19 vaccine candidate JNJ-78436735 also known as Ad26COV2S.
Johnson Johnsons coronavirus vaccine trial is the largest in the world. Johnson Johnsons Janssen COVID-19 Vaccine. The company enrolled up to 60000 volunteers across three continents to take their coronavirus vaccine.
CDC and FDA have recommended that use of Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine resume in the United States effective April 23 2021. Johnson Johnson announced last week that it would begin test trials with the COVID vaccine on children as well as on more vulnerable individuals such as pregnant women and those with underlying. Trials For 2 Dose Johnson Johnson Vaccine.
 Johnson Johnson Gets Approval For Phase Iii Covid 19 Trial In Spain
Johnson Johnson Gets Approval For Phase Iii Covid 19 Trial In Spain
 J J Pause Adds To Adenovirus Vaccine Doubts Evaluate
J J Pause Adds To Adenovirus Vaccine Doubts Evaluate
 Johnson Johnson Vaccine Shows 66 Efficacy For Moderate And Severe Covid Financial Times
Johnson Johnson Vaccine Shows 66 Efficacy For Moderate And Severe Covid Financial Times
 J J Enrolls About 45 000 Participants For Late Stage Covid 19 Vaccine Trial Reuters
J J Enrolls About 45 000 Participants For Late Stage Covid 19 Vaccine Trial Reuters
 Johnson Johnson Expands Covid 19 Vaccine Trial To Adolescents
Johnson Johnson Expands Covid 19 Vaccine Trial To Adolescents
 Backlash Against Johnson Johnson S Covid 19 Vaccine Is Real And Risky Here S How To Make Its Rollout A Success
Backlash Against Johnson Johnson S Covid 19 Vaccine Is Real And Risky Here S How To Make Its Rollout A Success
 5 Latest Facts About Johnson Johnson S Investigational Janssen Covid 19 Vaccine Candidate
5 Latest Facts About Johnson Johnson S Investigational Janssen Covid 19 Vaccine Candidate
Early Trial Results Show Promise For Johnson Johnson And Beth Israel Vaccine Commonhealth
 Johnson Johnson Vaccine Is 66 Effective In Global Study Politico
Johnson Johnson Vaccine Is 66 Effective In Global Study Politico
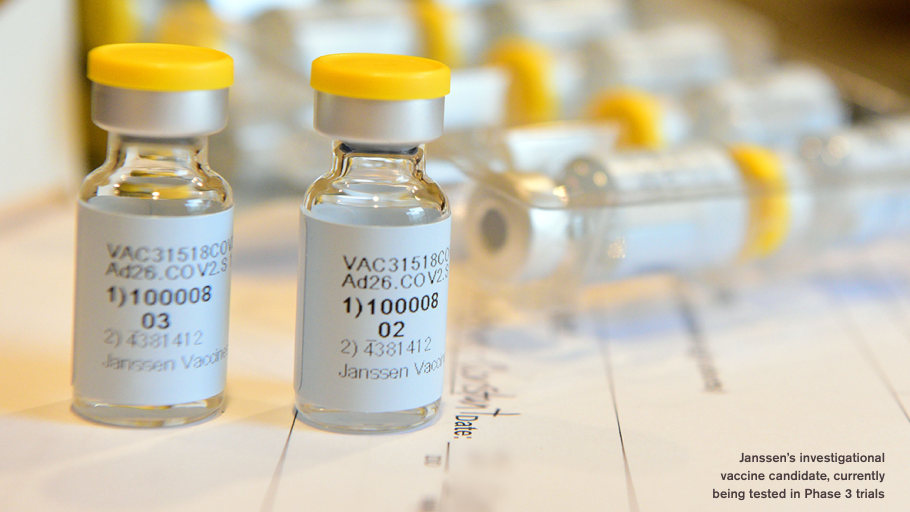 About Our Ensemble Studies Johnson Johnson
About Our Ensemble Studies Johnson Johnson

 Johnson Johnson Announces Single Shot Janssen Covid 19 Vaccine Candidate Met Primary Endpoints In Interim Analysis Of Its Phase 3 Ensemble Trial
Johnson Johnson Announces Single Shot Janssen Covid 19 Vaccine Candidate Met Primary Endpoints In Interim Analysis Of Its Phase 3 Ensemble Trial
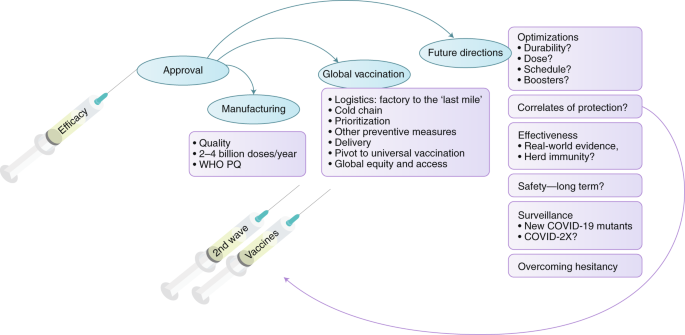 Looking Beyond Covid 19 Vaccine Phase 3 Trials Nature Medicine
Looking Beyond Covid 19 Vaccine Phase 3 Trials Nature Medicine
 Johnson Johnson Readies To Resume Covid 19 Vaccine Trial In The Us
Johnson Johnson Readies To Resume Covid 19 Vaccine Trial In The Us

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.